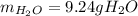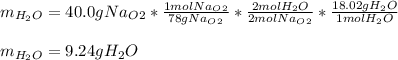Chemistry, 26.02.2021 09:20 milkshakegrande101
A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O) =18.02g/mol
M(NA2O2)= 78g/mol
2Na2O2 (s)+2h2O(I)—> 4NaOH(aq) + O2 (g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 22.06.2019 23:20, svaskeacevilles5477
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O)...
M(H2O)...
Questions in other subjects:

History, 25.06.2019 05:00




Mathematics, 25.06.2019 05:00


Mathematics, 25.06.2019 05:00



Biology, 25.06.2019 05:00