
Chemistry, 26.02.2021 07:40 alexandroperez13
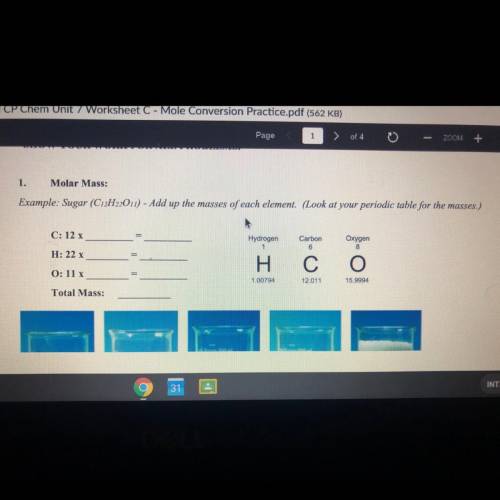
Example: Sugar (C12H22O11) - Add up the masses of each element. (Look at your periodic table for the masses.) Please and thank you so much :)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2
You know the right answer?
Example: Sugar (C12H22O11) - Add up the masses of each element. (Look at your periodic table for the...
Questions in other subjects:

English, 09.10.2019 19:30

History, 09.10.2019 19:30


Mathematics, 09.10.2019 19:30

Mathematics, 09.10.2019 19:30

Geography, 09.10.2019 19:30


Biology, 09.10.2019 19:30

Business, 09.10.2019 19:30



