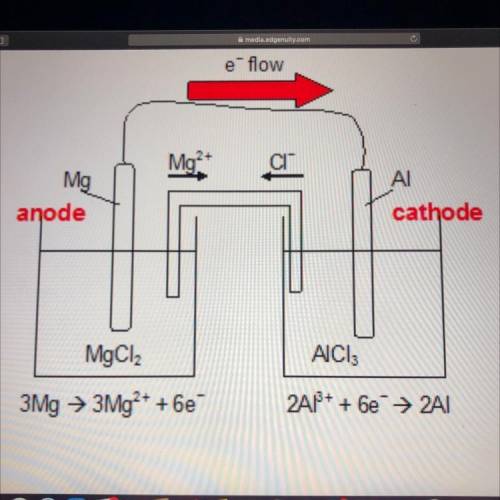
Look at the diagram of an electrochemical cell below.
Which observation would be most likely to happen if a power supply were added to the electrochemical cell?
A) Magnesium would be oxidized.
B) Electrons would flow through the salt bridge.
C) The AI electrode would become neutral.
D) The Mg electrode would become the cathode.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
Look at the diagram of an electrochemical cell below.
Which observation would be most likely to hap...
Questions in other subjects:

History, 21.04.2021 16:20

Mathematics, 21.04.2021 16:20

Mathematics, 21.04.2021 16:20


Computers and Technology, 21.04.2021 16:20


Mathematics, 21.04.2021 16:20

Advanced Placement (AP), 21.04.2021 16:20