
Chemistry, 25.02.2021 18:40 fluffyanimal456
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation below: Cu + 2AgNO3 →Cu(NO3)2 + 2Ag Calculate the number of moles of copper(II) nitrate produced when 5 moles of copper react. Type your answer as a number with 1 significant figure. Make sure to include the correct units in your answer. Units are a type of measurement i. e. gram (g) or mole (mol). Do not include the chemical formula in your answer.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

You know the right answer?
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation...
Questions in other subjects:

Spanish, 14.12.2020 06:10

Mathematics, 14.12.2020 06:10



Mathematics, 14.12.2020 06:10

Biology, 14.12.2020 06:10

Mathematics, 14.12.2020 06:10

Mathematics, 14.12.2020 06:10

Mathematics, 14.12.2020 06:10

German, 14.12.2020 06:10

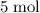 of
of 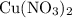 would be produced (assuming that reaction does not run out of
would be produced (assuming that reaction does not run out of 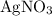 until all the
until all the 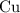 was converted.)
was converted.) 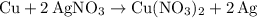 .
. . In other words, the actual equation for this reaction should be:
. In other words, the actual equation for this reaction should be: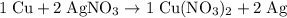 .
. .
. before running out of any other reactant.) This coefficient ratio would be equal to the ratio between:
before running out of any other reactant.) This coefficient ratio would be equal to the ratio between: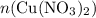 , the number of moles of
, the number of moles of 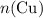 , the number of moles of
, the number of moles of 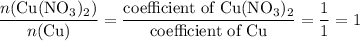 .
.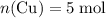 . Assume that
. Assume that 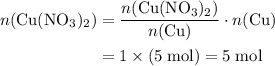 .
.

