
Chemistry, 25.02.2021 18:20 alejandr1872913
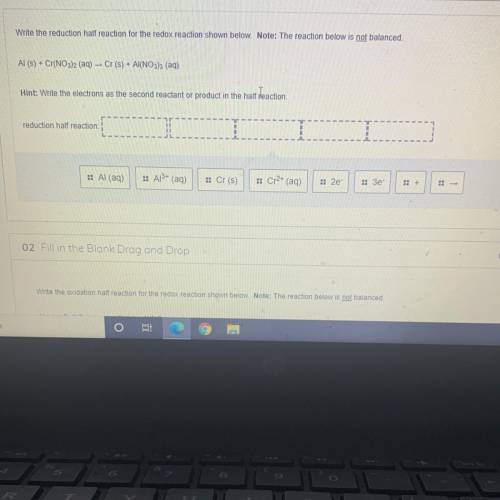
Write the reduction half reaction for the redox reaction shown below. Note: The reaction below is not balanced.
Al(s) + Cr(NO3)2 (aq) – Cr(s) + Al(NO3)3 (aq)
Hint: Write the electrons as the second reactant or product in the half reaction.
reduction half reaction:
:: Al (aq)
:: Al3+ (aq)
:: Cr(s)
:: Cr2+ (aq)
:: 2e
:: Зе-

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, sophiebeardsley94
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

You know the right answer?
Write the reduction half reaction for the redox reaction shown below. Note: The reaction below is no...
Questions in other subjects:

Mathematics, 04.11.2020 02:10



Mathematics, 04.11.2020 02:10

English, 04.11.2020 02:10


Mathematics, 04.11.2020 02:10

History, 04.11.2020 02:10




