HELP PLEASE...
_
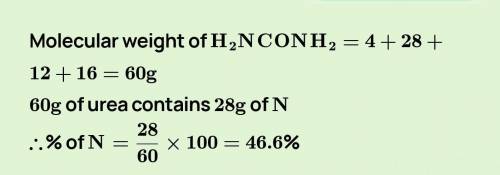
Calculate the percentage of nitrogen in urea CO(NH2)2.
(Atom...

Chemistry, 25.02.2021 14:00 shadowangel84
 HELP PLEASE...
HELP PLEASE...
_
Calculate the percentage of nitrogen in urea CO(NH2)2.
(Atomic mass: N=14, C=12, H=1)
_
all the best : D



Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 30.01.2020 17:03



Arts, 30.01.2020 17:03





Mathematics, 30.01.2020 17:03