Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 22:30, vhife4901
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
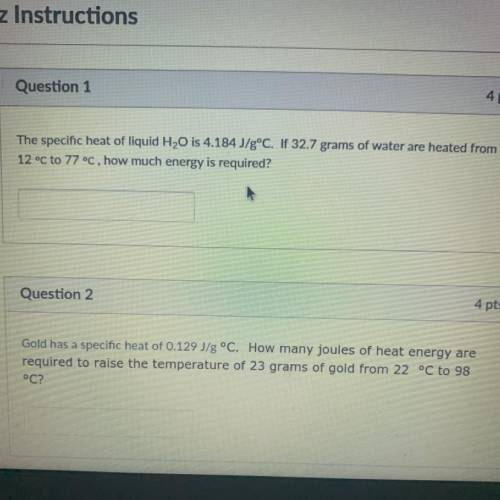
The specific heat of liquid H2O is 4.184 J/g°C. If 32.7 grams of water are heated from
12 °C to 77...
Questions in other subjects:


Mathematics, 04.04.2021 14:00

Physics, 04.04.2021 14:00



History, 04.04.2021 14:00

Health, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00