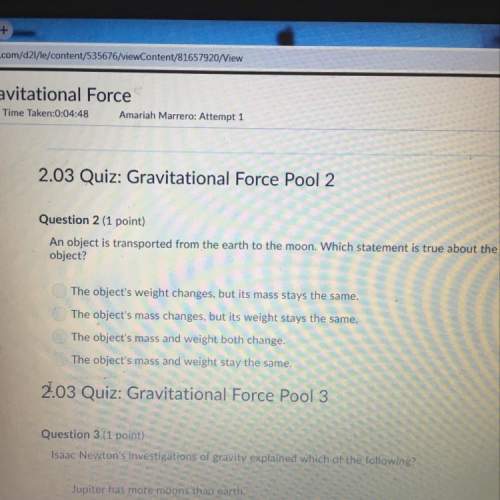
Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 07:30, SchoolFirst9811
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н, о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2
You know the right answer?
If 5 moles of carbon monoxide react with 2.3 moles of hydrogen gas, how many moles of methanol can b...
Questions in other subjects:

Mathematics, 24.06.2019 14:00


Mathematics, 24.06.2019 14:00

Mathematics, 24.06.2019 14:00


Mathematics, 24.06.2019 14:00