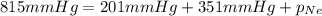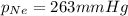A 2.00L mixture of helium, nitrogen, and neon has a total pressure of 815 mmHg at a
temperature of 255K. If the partial pressure of helium is 201 mmHg and the partial
pressure of nitrogen is 351 mmHg, what is the partial pressure of neon in the mixture?
O 709 mmHg
O 512 mmHg
O 667 mmHg
O 263 mmHg

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, 24lbriscoe
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1

Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1
You know the right answer?
A 2.00L mixture of helium, nitrogen, and neon has a total pressure of 815 mmHg at a
temperature of...
Questions in other subjects:





Geography, 16.11.2020 16:40





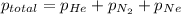
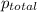 =total pressure of gases = 815 mm Hg
=total pressure of gases = 815 mm Hg
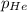 = partial pressure of helium = 201 mm Hg
= partial pressure of helium = 201 mm Hg
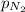 = partial pressure of nitrogen = 351 mm Hg
= partial pressure of nitrogen = 351 mm Hg
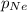 = partial pressure of Neon = ?
= partial pressure of Neon = ?