
Chemistry, 24.02.2021 21:30 CameronVand21
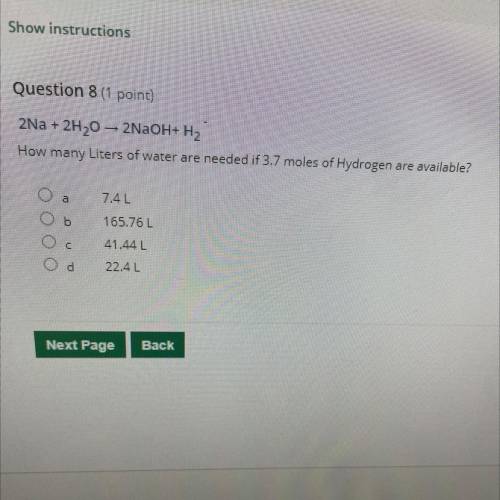
2Na + 2H20 – 2NaOH+H2 How many Liters of water are needed if 3.7 moles of Hydrogen are available?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
2Na + 2H20 – 2NaOH+H2
How many Liters of water are needed if 3.7 moles of Hydrogen are available?
<...
Questions in other subjects:

Chemistry, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Social Studies, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Biology, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01



