
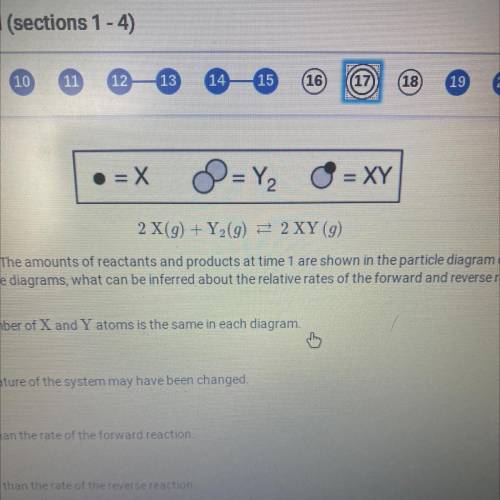
2 X(9) + Y (9) = 2XY (9)
A reversible reaction is represented by the equation above. The amounts of reactants and products at time 1 are shown in the particle diagram on the left. The particle diagram on the right shows the
amounts of reactants and products at time 2. Based on the diagrams, what can be inferred about the relative rates of the forward and reverse reactions between time 1 and time 2?
a. nothing can be inferred because the total number of X and Y atoms is the same
each diagram
b. Nothing can be inferred because the temperature of the system may have been changed
c. The rate of the reverse reaction is greater than the rate of the forward reaction
d. The rate of the forward reaction is greater than the rate of the reverse reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, PinkDivaGirl02
Compare these two waves : a. the blue wave has a higher pitch, but the orange wave is louder. b. the blue and orange waves have the same volume, but the blue wave has a higher pitch. c. the blue and orange waves have the same pitch, but the blue wave is louder. d. the orange wave has a higher pitch, but the blue wave is louder.
Answers: 1



You know the right answer?
2 X(9) + Y (9) = 2XY (9)
A reversible reaction is represented by the equation above. The amounts of...
Questions in other subjects:



Chemistry, 20.08.2021 16:10

Mathematics, 20.08.2021 16:10



Mathematics, 20.08.2021 16:10


French, 20.08.2021 16:10



