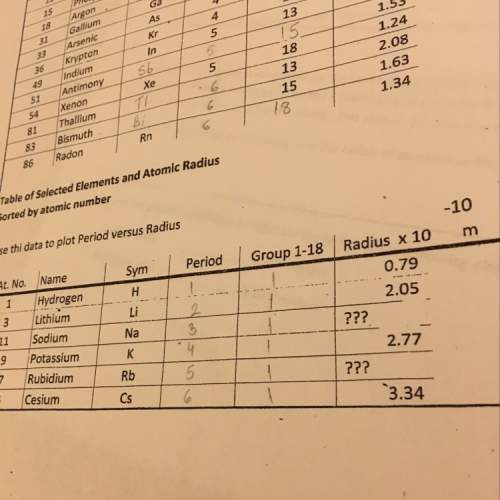
Stoichiometry:
S8 + 12 O2 → 8 SO3
How much sulfur trioxide (in grams) will form if 501....


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, sriggins1375
Which statement accurately describes the rock layers? layer 8 is older than layer 1. layer 3 is younger than layer 6. layer 4 and layer 10 are the same relative age. layer 2 and layer 9 are the same relative age.
Answers: 3

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Questions in other subjects:

Advanced Placement (AP), 09.05.2021 01:10







English, 09.05.2021 01:20