
Chemistry, 23.02.2021 09:20 sierradanielle9280
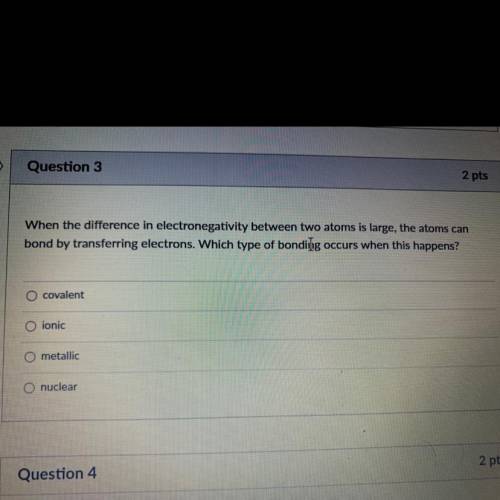
When the difference in electronegativity between two atoms is large, the atoms can
bond by transferring electrons. Which type of bonding occurs when this happens?
covalent
ionic
metallic
nuclear
ASAP quick

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
When the difference in electronegativity between two atoms is large, the atoms can
bond by transfer...
Questions in other subjects:



Mathematics, 19.10.2019 10:30

Mathematics, 19.10.2019 10:30


Chemistry, 19.10.2019 10:30



Advanced Placement (AP), 19.10.2019 10:30



