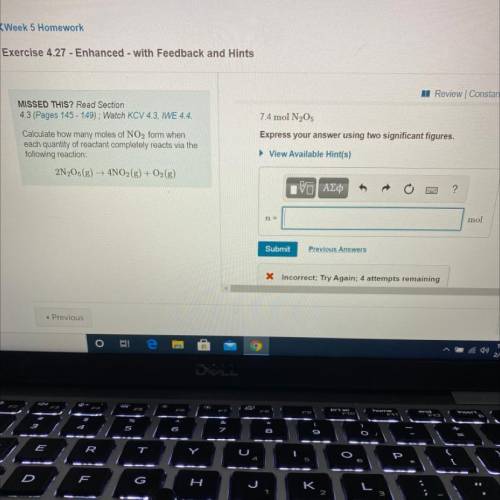
Calculate how many moles of NO2 form when
each quantity of reactant completely reacts via the
...

Chemistry, 23.02.2021 09:30 22MadisonT
Calculate how many moles of NO2 form when
each quantity of reactant completely reacts via the
following reaction:
2N205 (g) → 4NO2(g) + O2(g)
7.4 mol N2O5
Express your answer using two significant figures.
Will give brainliest! :)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Questions in other subjects:




Mathematics, 22.09.2021 08:20

Mathematics, 22.09.2021 08:20

Mathematics, 22.09.2021 08:20

Social Studies, 22.09.2021 08:20

History, 22.09.2021 08:20




