Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is...

Chemistry, 23.02.2021 09:20 keilajohnson810
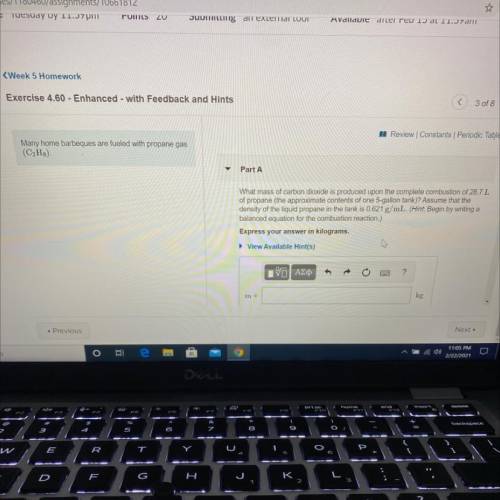
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is produced upon the complete combustion of 28.7L
of propane (the approximate contents of one 5-gallon tank)? Assume that the
density of the liquid propane in the tank is 0.621 g/mL.
Express your answer in kilograms.
Will give brainliest!

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1
You know the right answer?
Questions in other subjects:


SAT, 16.10.2020 06:01

Biology, 16.10.2020 06:01


Spanish, 16.10.2020 06:01



Health, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

Chemistry, 16.10.2020 06:01



