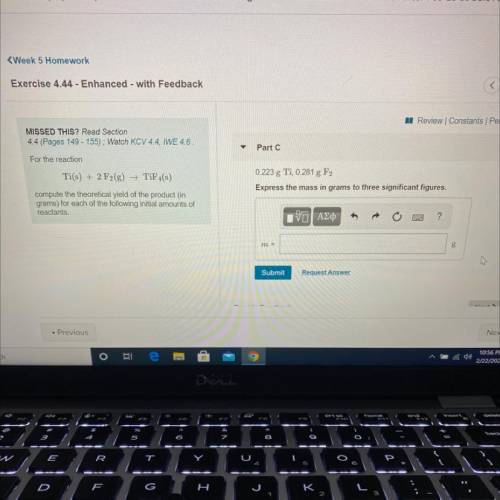
compute the theoretical yield of the product (in

For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
grams) for each of the following initial amounts of
reactants.
0.223g Ti, 0.281g F2
Express your answer using three significant figures.
please help! will give brainliest.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
compute the theoretical yield of the product (in
Questions in other subjects:

Mathematics, 15.01.2022 19:50

Mathematics, 15.01.2022 20:00




Mathematics, 15.01.2022 20:00

History, 15.01.2022 20:00





