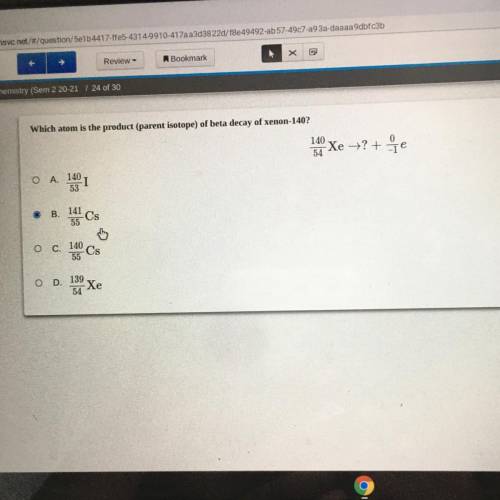
Which atom is the product (parent isotope) of beta decay of xenon-140?
140
Xe = ?+7e
54...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

You know the right answer?
Questions in other subjects:



History, 02.08.2019 22:00


Mathematics, 02.08.2019 22:00


Biology, 02.08.2019 22:00

English, 02.08.2019 22:00