Chemistry, 22.02.2021 23:50 live4dramaoy0yf9
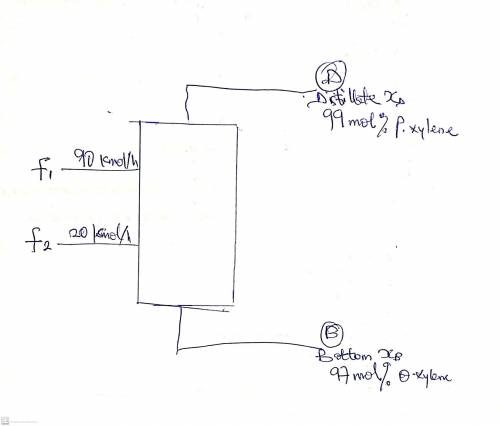
3. A very large distillation column is separating p-xylene (more volatile) from o-xylene. The column has two feeds that are saturated liquids. Feed 1 flows into the column at a rate of 90 kmol/h and contains 42 mol% p-xylene. Feed 2 flows at a rate of 20 kmol/h and contains 9 mol% p-xylene. The bottoms product should be 97 mol% o-xylene, and the distillate product should be 99 mol% p-xylene. Compute the distillate (D) and the bottoms (B) products flow rates

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
3. A very large distillation column is separating p-xylene (more volatile) from o-xylene. The column...
Questions in other subjects:



Computers and Technology, 19.11.2019 18:31