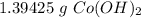Chemistry, 22.02.2021 23:30 Uhmjujiooo4220
Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2NaCl + Co(OH)₂
If you react 10.0 mL of 1.5 M CoCl₂ with plenty of NaOH, how many grams of Co(OH)₂ will precipitate out?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2


Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2Na...
CoCl₂ + 2NaOH → 2Na...
Questions in other subjects:

Biology, 07.11.2020 01:00



Mathematics, 07.11.2020 01:00



Health, 07.11.2020 01:00

Spanish, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

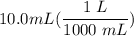 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 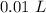 [DA] Find moles of CoCl₂ [Molarity]:
[DA] Find moles of CoCl₂ [Molarity]: 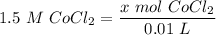 [DA] Solve for x [Multiplication Property of Equality]:
[DA] Solve for x [Multiplication Property of Equality]: 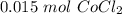 [DA] Set up [Reaction Stoich]:
[DA] Set up [Reaction Stoich]: 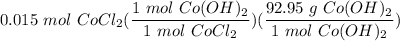 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: