
Chemistry, 30.09.2019 03:30 ConfusedJuliana
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775 ml of water. calculate the following:
a) moles of k2so4
b)millimoles of k2so4
c)molarity of k2so4, k+, so4(2-)
d)ppm of k2so4
e)%(w/v) k2so4
f)pk+
g)pso4(2-)

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775...
Questions in other subjects:




Mathematics, 09.04.2021 23:00

Spanish, 09.04.2021 23:00





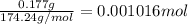

![[K_2SO_4]=\frac{0.001016 mol}{0.775 L}=0.001311 mol/L](/tpl/images/0275/4296/7b54d.png)
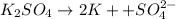
![[K^+]=2\times [K_2SO_4]=2\times 0.001311 mol/L=0.002622 mol/L](/tpl/images/0275/4296/87b7a.png)
![[SO_4^{2-}]=1\times [K_2SO_4]=1\times 0.001311 mol/L=0.001311 mol/L](/tpl/images/0275/4296/fd0e3.png)
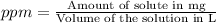
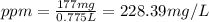
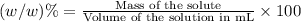
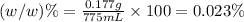
![pK^=-\log[K^+]](/tpl/images/0275/4296/a9ce4.png)
![pK^+=-\log[0.002622 M]=2.58](/tpl/images/0275/4296/0acce.png)
![pSO_4^{2-}=-\log[SO_4^{2-}]](/tpl/images/0275/4296/bd352.png)
![pK^+=-\log[0.001311 M]=2.88](/tpl/images/0275/4296/30697.png)



