
Chemistry, 22.02.2021 01:00 montgomerykarloxc24x
48g of methane was burned in an excess of air. What mass of carbon dioxide would be produced in the reaction assuming complete combustion? Use the information below to answer the question.
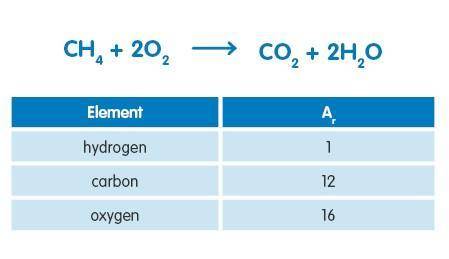

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, monnn91351
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1


Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3
You know the right answer?
48g of methane was burned in an excess of air. What mass of carbon dioxide would be produced in the...
Questions in other subjects:

Mathematics, 30.03.2021 20:20

Mathematics, 30.03.2021 20:20

Mathematics, 30.03.2021 20:20

Business, 30.03.2021 20:20

Mathematics, 30.03.2021 20:20

English, 30.03.2021 20:20



English, 30.03.2021 20:20

Mathematics, 30.03.2021 20:20




