
Chemistry, 21.02.2021 18:00 donaldwilliams31
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aqueous solution of H2S04. (IF = 96500C, Volume at s. t.p = 22.4
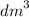

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aque...
Questions in other subjects:



Computers and Technology, 10.11.2020 23:30


History, 10.11.2020 23:30

Mathematics, 10.11.2020 23:30

Mathematics, 10.11.2020 23:30

Mathematics, 10.11.2020 23:30




