CHEMISTRY SYNTHESIS OF CALCIUM CARBONATE
INTRODUCTION
LABORATORY SIMULATION
1) In this...

Chemistry, 21.02.2021 06:00 lorenaandreahjimenez
CHEMISTRY SYNTHESIS OF CALCIUM CARBONATE
INTRODUCTION
LABORATORY SIMULATION
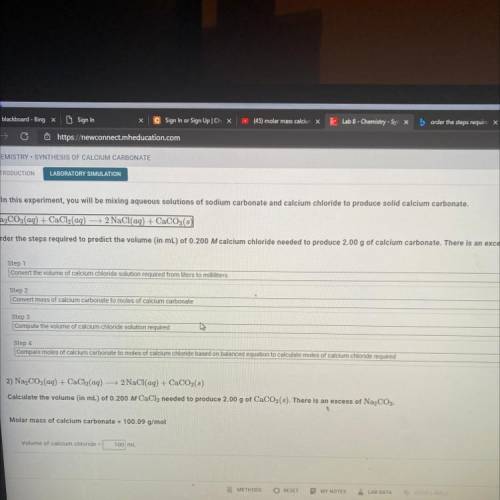
1) In this experiment, you will be mixing aqueous solutions of sodium carbonate and calcium chloride to produce solid calcium carbonate.
Na2CO3(aq) + CaCl2(aq) → 2 NaCl(aq) + CaCO3(s)
Order the steps required to predict the volume (in mL) of 0.200 M calcium chloride needed to produce 2.00 g of calcium carbonate. There is al
Step 1
Convert the volume of calcium chloride solution required from liters to milliliters
Step 2
Convert mass of calcium carbonate to moles of calcium carbonate
Step 3
Compute the volume of calcium chloride solution required
Step 4
Compare moles of calcium carbonate to moles of calcium chloride based on balanced equation to calculate moles of calcium chloride required
2) Na2CO3(aq) + CaCl2(aq) 2 NaCl(aq) + CaCO3(8)
Calculate the volume (in mL) of 0.200 M CaCl2 needed to produce 2.00 g of CaCO3(3). There is an excess of Na2CO3.
Molar mass of calcium carbonate = 100.09 g/mol
Volume of calcium chloride =
100 ml
METHODS
RESET
MY NOTES
LAB DATA

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 04:20, monifaWilson
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 12.05.2021 07:00



Business, 12.05.2021 07:00

Spanish, 12.05.2021 07:00

Biology, 12.05.2021 07:00

Mathematics, 12.05.2021 07:00

Mathematics, 12.05.2021 07:00





