
Chemistry, 20.02.2021 05:30 malikvanison
Question 11:
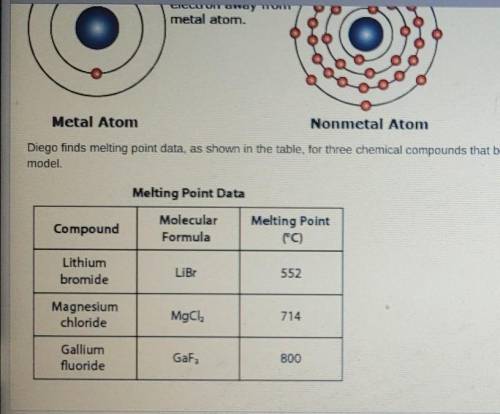
Diego claims that ions with greater amounts of charge have stronger interactions with other ions. Analyze the melting point data provided to determine whether the data support Diego's claim. Use the drop-down menus to BEST complete the analysis.
A stronger interaction between the ions in a compound can be inferred from a [blank] melting point.
The charges on the metal ions lithium, magnesium, and gallium for the compounds listed in the melting point data table are [blank] , [blank] , and [blank] , respectively.
The melting point data show that when the amount of charge on a metal ion is [blank] the melting point is higher.
Therefore, Diego's claim [blank] supported by the melting a metal ion is point data.
Answer Choices:
First Blank: Higher/Lower
Second, Third, and Four Blank: -3, -2, -1, 0, +1, +2, +3
Fifth Blank: Greater/Less
Sixth Blank: Is/Is Not

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Question 11:
Diego claims that ions with greater amounts of charge have stronger interactions with...
Questions in other subjects:

Mathematics, 02.09.2019 05:30



Chemistry, 02.09.2019 05:30

Mathematics, 02.09.2019 05:30


Computers and Technology, 02.09.2019 05:30

Mathematics, 02.09.2019 05:30


Social Studies, 02.09.2019 05:30



