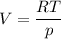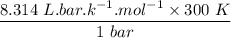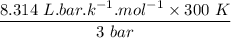Chemistry, 19.02.2021 17:00 bunbun2913
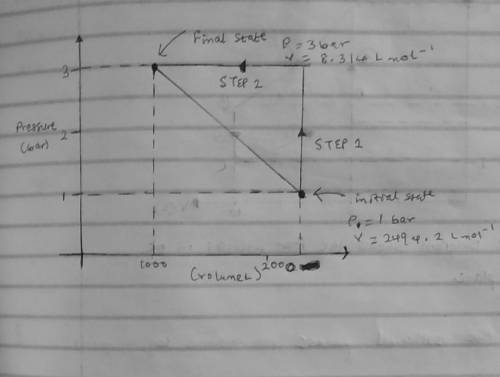
An ideal gas that is confined in piston-cylinder assembly (i. e., closed system) goes from an initial state of 1 bar at 300 K to a final state of 3 bar at 300 K by the following two-step process.
Process Path.
(Step 1) Heating at constant volume, and then
( Step 2) Cooling by holding the pressure constant.
Required:
a. Determine the initial and final molar mass.
b. Illustrate the two paths on a pressure-volume diagram. Clearly label the initial and final states, process steps and the direction of each step in the diagram.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 23.06.2019 18:30, adajadavis2843
What is the solution to the problem to the correct number of significant figures (102,900/12)+(170•1.27)
Answers: 1

Chemistry, 23.06.2019 18:30, suselygonza
The first step in creating a budget is a) getting a bank account. b) cutting back on expenses. c) knowing what your income is. d) listing all of your expenses.
Answers: 1

Chemistry, 23.06.2019 19:10, Kameon
Adna specialist is determining the probability of a random match using codis str markers vwhat amount of str markers dna specialist is determining the probability of a random match using would give the best probability of a match? a. 2 b. 5 c. 10 d. 15
Answers: 2
You know the right answer?
An ideal gas that is confined in piston-cylinder assembly (i. e., closed system) goes from an initia...
Questions in other subjects:



Mathematics, 26.08.2019 18:40

Mathematics, 26.08.2019 18:40




Mathematics, 26.08.2019 18:40


Mathematics, 26.08.2019 18:40