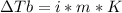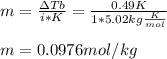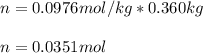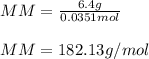Chemistry, 19.02.2021 09:30 marlandwilliams10
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0.49 K higher than the boiling point of pure CC14. Calculate the molar mass of
adrenaline. (K = 5.02 kg K/mol).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1



Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0...
Questions in other subjects:


Biology, 27.01.2020 20:31

Biology, 27.01.2020 20:31

Mathematics, 27.01.2020 20:31

Mathematics, 27.01.2020 20:31


Mathematics, 27.01.2020 20:31


Mathematics, 27.01.2020 20:31