
Chemistry, 18.02.2021 09:20 kaylamount
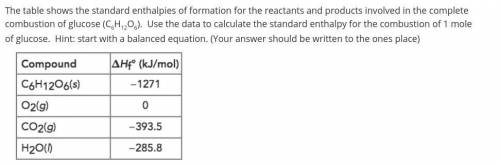
The table shows the standard enthalpies of formation for the reactants and products involved in the complete combustion of glucose (C6H12O6). Use the data to calculate the standard enthalpy for the combustion of 1 mole of glucose. Hint: start with a balanced equation. (Your answer should be written to the ones place)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
The table shows the standard enthalpies of formation for the reactants and products involved in the...
Questions in other subjects:

Chemistry, 14.05.2021 23:50


English, 14.05.2021 23:50



Mathematics, 14.05.2021 23:50

Mathematics, 14.05.2021 23:50






