
Chemistry, 11.10.2019 18:50 cadeedmiston
Using the periodic table, explain the difference between hydrogen, deuterium, and tritium - i. e. hydrogen-1, hydrogen-2, and hydrogen-3.
a) allotropes vary in their number of protons - so they contain 1, 2, and 3 protons, respectively.
b) the atoms deviate in their number of photons - so they contain 1, 2, and 3 photons, respectively.
c) isotopes differ only in their number of neutrons - so they contain 0, 1, and 2 neutrons, respectively.
d) these configurations diverge in their number of electrons - so they contain 1, 2, and 3 electrons, respectively.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
Using the periodic table, explain the difference between hydrogen, deuterium, and tritium - i. e. hy...
Questions in other subjects:


Mathematics, 20.09.2021 08:10

English, 20.09.2021 08:10




Mathematics, 20.09.2021 08:10

Arts, 20.09.2021 08:10

Mathematics, 20.09.2021 08:10

Mathematics, 20.09.2021 08:10

 , deuterium
, deuterium 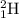 , and tritium
, and tritium  .
.

