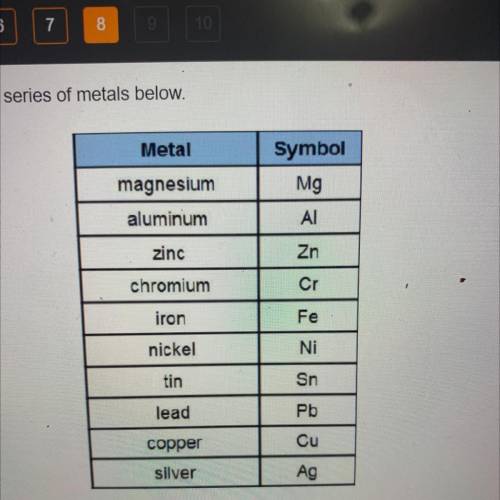
The following reaction occurs in an electrochemical cell.
Sn2++ Pb → Pb2++ Sn
What type of el...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

You know the right answer?
Questions in other subjects:


Geography, 24.10.2021 02:00






Mathematics, 24.10.2021 02:00

Biology, 24.10.2021 02:00

Arts, 24.10.2021 02:00