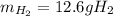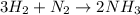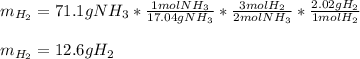Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Of nitrogen gas)?
How many grams of H2 are needed to produce 71.1 g of ammonia (NH3) (assuming unli...
Questions in other subjects:


Mathematics, 21.02.2020 10:47

Mathematics, 21.02.2020 10:48

English, 21.02.2020 10:50


English, 21.02.2020 10:50

Mathematics, 21.02.2020 10:51

Mathematics, 21.02.2020 10:52

Chemistry, 21.02.2020 10:52