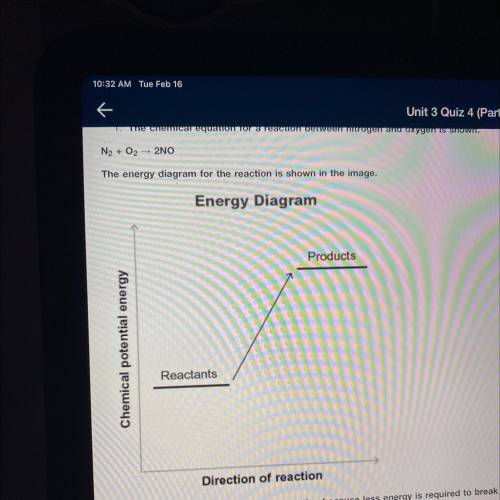
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
T...

Chemistry, 16.02.2021 21:50 brookie125
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
Then energy diagram for the reaction is shown in the image.
A) energy is absorbed, less energy, break bonds, form new bonds.
B) energy is released, more energy, break bonds, form new bonds
C) Energy is absorbed, the bond energy of the reactant is higher than the bond energy of the products
D) Energy is released, the bond energy of the reactant is lower than the bond energy of the products

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Questions in other subjects:

Chemistry, 20.05.2021 18:10


Mathematics, 20.05.2021 18:10



Mathematics, 20.05.2021 18:10


Mathematics, 20.05.2021 18:10

Mathematics, 20.05.2021 18:10




