
Chemistry, 16.02.2021 18:50 screamqueen
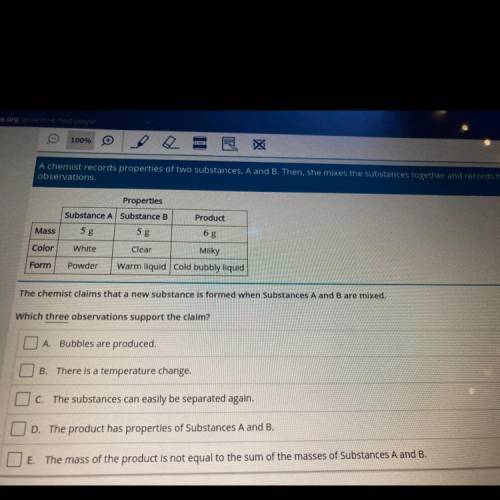
A chemist records properties of two substances, A and B. Then, she mixes the substances together and records her
observations.
Product
Properties
Substance A Substance B
5 g
5 g
White
Clear
Mass
Color
Milky
Form
Powder
Warm liquid cold bubbly liquid
The chemist claims that a new substance is formed when Substances A and B are mixed.
Which three observations support the claim?
O A Bubbles are produced.
B
There is a temperature change.
O c. The substances can easily be separated again.
OD. The product has properties of Substances A and B.
O E. The mass of the product is not equal to the sum of the masses of Substances A and B.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
A chemist records properties of two substances, A and B. Then, she mixes the substances together and...
Questions in other subjects:

History, 24.02.2021 23:20




English, 24.02.2021 23:20




Mathematics, 24.02.2021 23:20

Chemistry, 24.02.2021 23:20



