
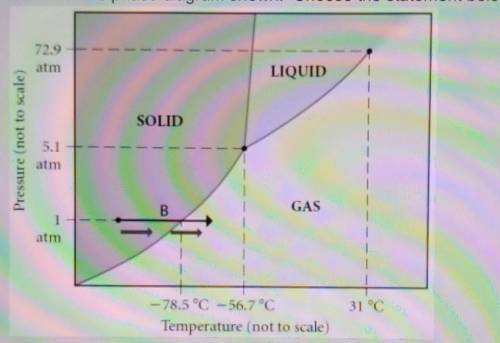
Select one: a. The line separating the solid and liquid phases represents the H vap b. The triple point of this substance occurs at a temperature of 31°C. c. The solid phase of this substance is higher in density than the liquid phase. d. At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. e. None of the above are true. which is true?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
You know the right answer?
Select one: a. The line separating the solid and liquid phases represents the H vap b. The triple po...
Questions in other subjects:

Arts, 23.10.2019 04:00

Social Studies, 23.10.2019 04:00


Mathematics, 23.10.2019 04:00

Mathematics, 23.10.2019 04:00

History, 23.10.2019 04:00




Mathematics, 23.10.2019 04:00



