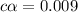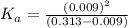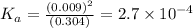Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, bananaslugs
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

You know the right answer?
In the laboratory, a general chemistry student measured the pH of a 0.313 M aqueous solution of acet...
Questions in other subjects:



Mathematics, 15.02.2021 21:50






Biology, 15.02.2021 21:50

Mathematics, 15.02.2021 21:50

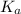 for the acid is
for the acid is 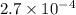
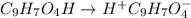
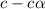
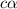
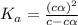
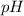 = 2.031
= 2.031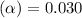
![[H^+]=c\times \alpha](/tpl/images/1121/0617/4fc41.png)
![[H^+]=0.500\times 0.030=0.015](/tpl/images/1121/0617/28636.png)
![pH=-log[H^+]](/tpl/images/1121/0617/15713.png)
![2.031=-log[H^+]](/tpl/images/1121/0617/0b1a6.png)
![[H^+]=0.009](/tpl/images/1121/0617/13241.png)
![[H^+]=c\alpha](/tpl/images/1121/0617/21a04.png)