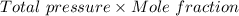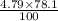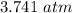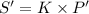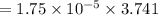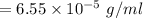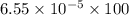Chemistry, 15.02.2021 22:20 giraffesaur44
Nitrogen, , is soluble in blood and can cause intoxication at sufficient concentration. For this reason, the U. S. Navy advises divers using compressed air not to go below 125 feet. The total pressure at this depth is 4.79 atm. If the solubility of nitrogen at 1.00 atm is g/100 mL of water, and the mole percent of nitrogen in air is 78.1, what is the solubility of nitrogen in water from air at 4.79 atm

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, fgcherubin
Consider the balanced equation below. n2h4 + 2h2o2 n2 + 4h2o what are the mole ratios of hydrazine (n2h4) to hydrogen peroxide (h2o2) and hydrazine to water? 1: 2 and 1: 4 1: 3 and 1: 4 1: 2 and 3: 5 1: 3 and 3: 5
Answers: 3

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

You know the right answer?
Nitrogen, , is soluble in blood and can cause intoxication at sufficient concentration. For this rea...
Questions in other subjects:

English, 10.11.2020 04:40

Spanish, 10.11.2020 04:40

Mathematics, 10.11.2020 04:40




Business, 10.11.2020 04:40

Mathematics, 10.11.2020 04:40


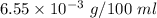 "
"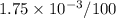
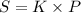
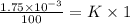
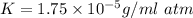 (Henry's constant)
(Henry's constant)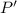 ) will be:
) will be: