
Chemistry, 15.02.2021 22:10 marandahuber
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to produced Ammonium. Which of the reacted will be in excess and by how many moles?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, pennygillbert
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1


Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:30, jarteria0
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to p...
Questions in other subjects:





Mathematics, 26.02.2022 05:30





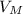 = 22, 4 l/mol
= 22, 4 l/mol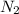 ) = 6,72
) = 6,72 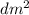 = 6,72 l
= 6,72 l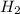 ) = 6,72
) = 6,72 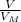
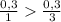 (we have more hydrogen than is need for the reaction)
(we have more hydrogen than is need for the reaction)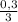 = 0,1 mol (
= 0,1 mol (

