
Chemistry, 15.02.2021 20:30 madisonsimmons1120
Find the concentration of the saline solution in terms of % by mass using your exact mass of NaCl, the volume of water (80.0 mL), and density of water being 0.9981 g/mL
1) Calculate the predicted (theoretical) concentration of the saline solution in terms of % by mass using your exact mass of NaCl, the volume of water (80.0 mL), and density of water being 0.9981 g/mL. Show the calculation in detail with the corresponding units of measurement and the appropriate sig. figs. The mass of NaCl used was 17.518 g.
2) Use the information to calculate the experimental value for the concentration (% by mass) of your saline solution based on your experimental value for density obtained with the volumetric pipette. You must show ALL the work and the calculation leading to the result in detail for full credit. The graph equation given is y=(0.0076x) + (0.9935) where y= density, x= Concentration and the density of solution using one experimental value for volumetric pipette;1.08

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:10, danielahchf
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2
You know the right answer?
Find the concentration of the saline solution in terms of % by mass using your exact mass of NaCl, t...
Questions in other subjects:


Geography, 27.07.2019 06:30

Mathematics, 27.07.2019 06:30

Geography, 27.07.2019 06:30

English, 27.07.2019 06:30

Geography, 27.07.2019 06:30


Geography, 27.07.2019 06:30


Geography, 27.07.2019 06:30

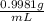
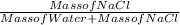 x 100%
x 100%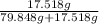 x 100%
x 100% 

