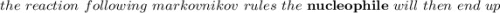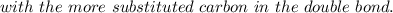Chemistry, 15.02.2021 19:50 elissadiaz15
According to Markovnikov's rule of the electrophilic addition to an alkene, the electrophile, usually a proton, is more likely to add to the in a double bond. This arrangement places the intermediate carbocation on the , which stabilizes it with the presence of . In the major product of a reaction following Markovnikov's rule, the will then end up on the more-substituted carbon in a double

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
You know the right answer?
According to Markovnikov's rule of the electrophilic addition to an alkene, the electrophile, usuall...
Questions in other subjects:

Mathematics, 07.04.2021 22:00



Mathematics, 07.04.2021 22:00



Physics, 07.04.2021 22:00


Social Studies, 07.04.2021 22:00

Mathematics, 07.04.2021 22:00