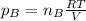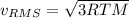Chemistry, 15.02.2021 19:40 mikehager4321
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Which of the two gases exerts the greater partial pressure?
b. The molecules or atoms of which gas have the greater average velocity?
c. The molecules of which gas have the greater average kinetic energy?
d . If a small hole were opened in the flask, which gas effuses more quickly?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 21.06.2019 17:10, PSBSolarYT
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Whi...
Questions in other subjects:




Mathematics, 31.07.2019 05:30

SAT, 31.07.2019 05:30