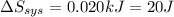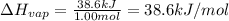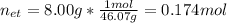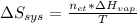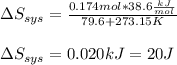Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
It takes 38.6 kJ of energy to vaporize 1.00 mol of ethanol (MW: 46.07 g/mol). What will be ΔSsys for...
Questions in other subjects:

English, 12.02.2021 14:10

Computers and Technology, 12.02.2021 14:10

History, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10

Biology, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10