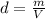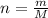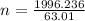Chemistry, 14.02.2021 14:00 patrick171888
A nitric acid solution containing 71.0% HNO3 (by mass) is available in a laboratory. How many moles of HNO3 are present in 1.98 L of this solution?
a.
62.8 mol
b.
31.7 mol
c.
22.3 mol
d.
0.0316 mol
e.
none of these

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
A nitric acid solution containing 71.0% HNO3 (by mass) is available in a laboratory. How many moles...
Questions in other subjects:



History, 13.09.2019 22:10


Social Studies, 13.09.2019 22:10

Mathematics, 13.09.2019 22:10

History, 13.09.2019 22:10

Chemistry, 13.09.2019 22:10


Mathematics, 13.09.2019 22:10