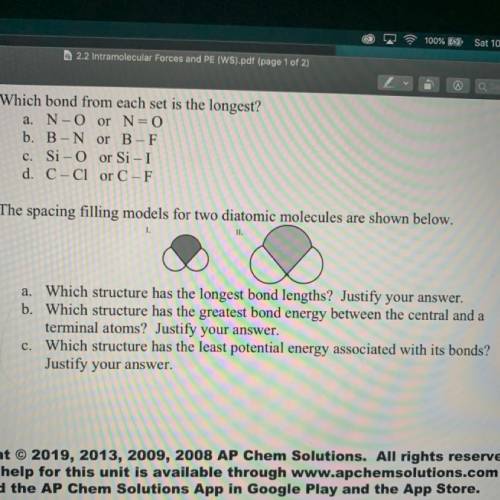
5) The spacing filling models for two diatomic molecules are shown below.
1.
II.
a. Whi...

Chemistry, 14.02.2021 06:20 Emilybaez15
5) The spacing filling models for two diatomic molecules are shown below.
1.
II.
a. Which structure has the longest bond lengths? Justify your answer.
b. Which structure has the greatest bond energy between the central and a
terminal atoms? Justify your answer.
c. Which structure has the least potential energy associated with its bonds?
Justify your answer.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 03.12.2021 09:20





Mathematics, 03.12.2021 09:20

Mathematics, 03.12.2021 09:20

Mathematics, 03.12.2021 09:20


Mathematics, 03.12.2021 09:20



