
Chemistry, 14.02.2021 01:20 NateTheBeast12
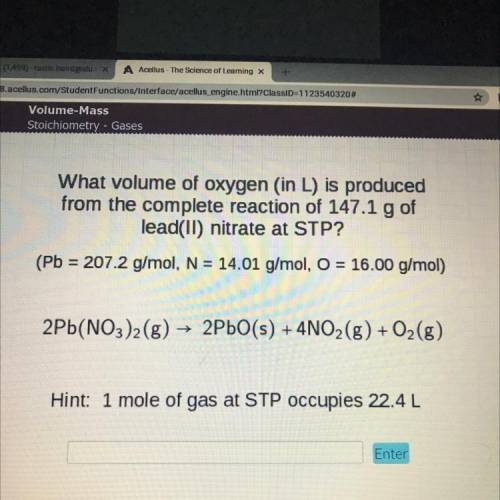
What volume of oxygen (in L) is produced
from the complete reaction of 147.1 g of
lead(II) nitrate at STP?
(Pb = 207.2 g/mol, N = 14.01 g/mol, O = 16.00 g/mol)
2Pb(NO3)2(g) → 2PbO(s) + 4NO2(g) + O2(g)
Hint: 1 mole of gas at STP occupies 22.4L

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

You know the right answer?
What volume of oxygen (in L) is produced
from the complete reaction of 147.1 g of
lead(II) ni...
lead(II) ni...
Questions in other subjects:

Mathematics, 14.09.2020 06:01

Social Studies, 14.09.2020 06:01

English, 14.09.2020 06:01

History, 14.09.2020 06:01

Social Studies, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01

Physics, 14.09.2020 06:01

Mathematics, 14.09.2020 06:01



