
Chemistry, 12.02.2021 19:30 edgartorres5123
Which is true about the compounds carbon monoxide (CO) and carbon dioxide (CO2)?
A. The compounds have the same properties because they contain the same elements.
B. The compounds have different properties because they are found in different amounts in the atmosphere.
C. The compounds have the same properties because they are both gases.
D. The compounds have different properties because they contain the same elements in different quantities.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
Which is true about the compounds carbon monoxide (CO) and carbon dioxide (CO2)?
A. The compounds h...
Questions in other subjects:

Mathematics, 17.07.2020 22:01





Mathematics, 17.07.2020 22:01

Social Studies, 17.07.2020 22:01


Mathematics, 17.07.2020 22:01

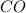 contains carbon and oxygen in the ratio of 3: 4 by mass whereas
contains carbon and oxygen in the ratio of 3: 4 by mass whereas 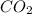 contains carbon and oxygen in the ratio of 3: 8 by mass.
contains carbon and oxygen in the ratio of 3: 8 by mass.


