Name
Virtual Chemistry
Unit 7 HW 4
Answer questions 1-12 about the phase diagram shown...

Chemistry, 12.02.2021 18:30 gabriellabadon2
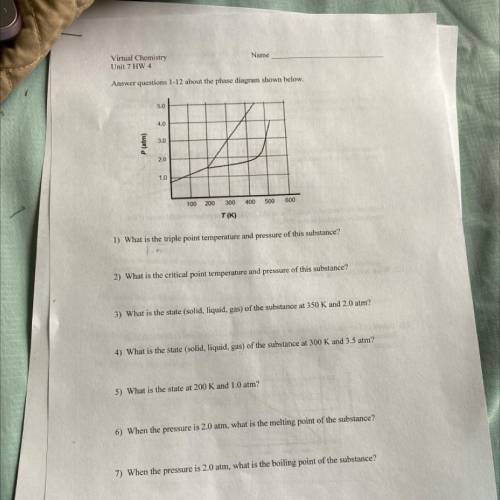
Name
Virtual Chemistry
Unit 7 HW 4
Answer questions 1-12 about the phase diagram shown below.
5.0
4.0
3.0
P (atm)
2.0
1.0
100 200
300
400
500
600
T(K)
1) What is the triple point temperature and pressure of this substance?
2) What is the critical point temperature and pressure of this substance?
3) What is the state (solid, liquid, gas) of the substance at 350 K and 2.0 atm?
4) What is the state (solid, liquid, gas) of the substance at 300 K and 3.5 atm?
5) What is the state at 200 K and 1.0 atm?
6) When the pressure is 2.0 atm, what is the melting point of the substance?
7) When the pressure is 2.0 atm, what is the boiling point of the substance?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, marknjenbennetp3j1v1
Listen base your answer to the question on the information below. propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below. c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol. what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1
You know the right answer?
Questions in other subjects:

Business, 02.02.2020 21:52

Biology, 02.02.2020 21:52


English, 02.02.2020 21:52


Mathematics, 02.02.2020 21:52






