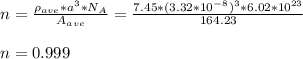Chemistry, 12.02.2021 07:20 senituliii
Some hypothetical alloy is composed of 25 wt% of metal A and 75 wt% of metal B. If the densities of metals A and B are 6.17 and 8.00 g/cm3 , respectively, and their respective atomic weights are 171.3 and 162.0 g/mol, determine whether the crystal structure for this alloy is simple cubic, facecentered cubic, or body-centered cubic. Assume a unit cell edge length of 0.332 nm

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

You know the right answer?
Some hypothetical alloy is composed of 25 wt% of metal A and 75 wt% of metal B. If the densities of...
Questions in other subjects:

Mathematics, 29.11.2021 08:20


Chemistry, 29.11.2021 08:20

Mathematics, 29.11.2021 08:20



Mathematics, 29.11.2021 08:20

Mathematics, 29.11.2021 08:20