
Chemistry, 12.02.2021 07:20 winterblackburn78
In ideal gas equation calculations, expressing pressure in Pascals (Pa), necessitates the use of the gas constant, R, equal to . In ideal gas equation calculations, expressing pressure in Pascals (Pa), necessitates the use of the gas constant, R, equal to . 8.314 m3-Pa/mol-K 0.08206 atm L mol-1K-1 1.987 cal mol-1K-1 62.36 L torr mol-1K-1 none of the above

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

You know the right answer?
In ideal gas equation calculations, expressing pressure in Pascals (Pa), necessitates the use of the...
Questions in other subjects:


Biology, 10.05.2020 19:57


Mathematics, 10.05.2020 19:57


Mathematics, 10.05.2020 19:57


Biology, 10.05.2020 19:57


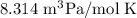
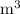
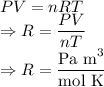
 is used so the gas constant value that must be used is
is used so the gas constant value that must be used is 


