
Chemistry, 19.09.2019 18:30 woodfordmaliky
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(aq)+3h2(g) (deltah)= -725.0 kj
2. hcl(> hcl(aq) (deltah)= -74.8kj 3. h2(g)+cl2(g) --> 2hcl(g) (deltah)=-1845.0kj 4. mcl3(s) --> mcl3(aq) (deltah)= -476.0kj use the information above to determine the enthalpy of the following reaction.
2m(s)+3cl2(g) > 2mcl3(s) (deltah) = kj

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, Mercedes12152002
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1


You know the right answer?
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
Questions in other subjects:

Mathematics, 29.01.2020 13:45

History, 29.01.2020 13:45

Mathematics, 29.01.2020 13:45

English, 29.01.2020 13:45


Mathematics, 29.01.2020 13:45

Mathematics, 29.01.2020 13:45

History, 29.01.2020 13:45

History, 29.01.2020 13:45

Chemistry, 29.01.2020 13:45

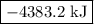
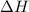 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.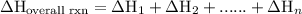
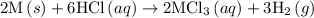 ...… (1)
...… (1)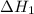 is -725 kJ.
is -725 kJ.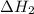 .
.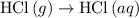 ...... (2)
...... (2) 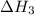 .
.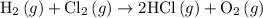 …… (3)
…… (3)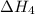 .
.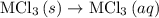 …… (4)
…… (4)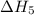 .
.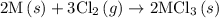 …… (5)
…… (5)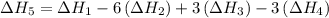 ...... (7)
...... (7) 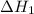 , -74.8 kJ for
, -74.8 kJ for 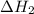 and -1845 kJ for
and -1845 kJ for 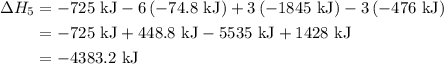
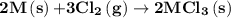 is -4383.2 kJ.
is -4383.2 kJ.

