
Chemistry, 12.02.2021 05:00 blakesmith0110
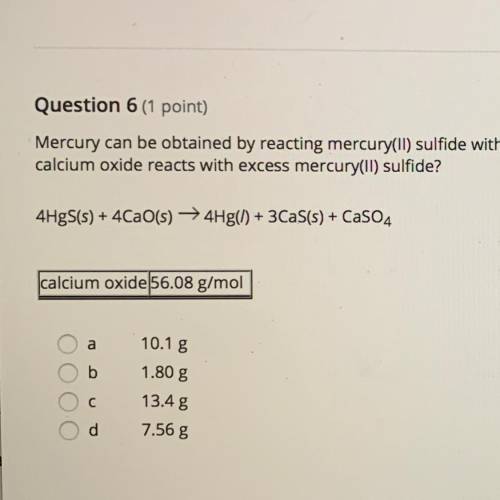
Mercury can be obtained by reacting mercury(II) sulfide with calcium oxide. How many grams of Mercury metal are produced when 2.11 g of calcium oxide reacts with excess mercury(II) sulfide?
4HgS+4CaO=4Hf+3CaS+CaSO4
a. 10.1 g
b. 1.80 g
c. 13.4 g
d. 7.56 g

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Mercury can be obtained by reacting mercury(II) sulfide with calcium oxide. How many grams of Mercur...
Questions in other subjects:


Social Studies, 08.01.2022 14:00

Mathematics, 08.01.2022 14:00


Mathematics, 08.01.2022 14:00


English, 08.01.2022 14:00



Mathematics, 08.01.2022 14:00



