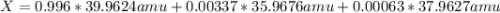Argon has three naturally-occurring isotopes: 99.6% of 40Ar, with an atomic weight of 39.9624 amu, 0.337% of 36Ar, with an atomic weight of 35.9676 amu, and 0.063% of 38Ar, with an atomic weight of 37.9627 amu. Calculate the average atomic weight of Ar. Round off the answer to six significant figures. Do not include the units.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
Argon has three naturally-occurring isotopes: 99.6% of 40Ar, with an atomic weight of 39.9624 amu, 0...
Questions in other subjects:


English, 24.07.2019 14:30

History, 24.07.2019 14:30

Biology, 24.07.2019 14:30


History, 24.07.2019 14:30